Could We Be Looking at a Vaccine Glut?

The first two months of the US’s COVID-19 vaccination campaign saw demand far exceeding available supply, a mismatch that generated widespread frustration. However, the next three months might be about the slow pace of administration leaving millions of available doses on shelves already strained from a massive stockpile of unused inventory.
As of March 4, the three manufacturers granted emergency use authorizations (EUAs) by the Food and Drug Administration—Pfizer/BioNTech, Moderna, and Johnson & Johnson—had made available 109.9 million doses of their COVID-19 vaccines, up 18.2 million from one week earlier. Over that same week, the U.S. administered 14.3 million shots. In other words, the number of unused shots—sitting in freezers or on shelves somewhere in the nation’s vast distribution network—increased by 3.9 million in one week.
The discrepancy between incoming supply and outgoing shots could grow dramatically in the coming weeks if the nation’s vaccination campaign—the complex logistical dance of getting the doses from where they are stored and into the arms of Americans—does not step up the pace. Between now and the end of May, the approved manufacturers have committed to delivering just under 400 million additional vaccines to the U.S. At the current pace of daily vaccinations, a substantial amount of that inventory will be unused for months.
The White House has been appropriately aggressive in pushing the manufacturers to speed up production. Last week, President Biden announced that his administration had worked with Johnson & Johnson to accelerate the delivery of the full 100 million doses purchased by the U.S. government by one month, from June 30 to May 31, and 20 million of the Johnson & Johnson doses are expected to ship in March.
With this sped-up schedule from Johnson & Johnson, the U.S. is now on track to receive sufficient doses to vaccinate 300 million Americans, with all of the needed supply in hand by the end of May. The Johnson & Johnson one-shot vaccine would inoculate 100 million people, while the Pfizer-BioNTech partnership and Moderna have committed to deliver a total of 400 million doses by the same deadline, or enough to give two shots to 200 million people. Thus, the combined production commitment from the three vaccine sponsors is 500 million doses, of which nearly 110 million have already been delivered.
As the supply outlook improves, the focus needs to shift to stepping up capacity for daily vaccinations. Over the past week, the average daily rate has been just more than 2 million shots per day, or 14 million per week. Based on the commitments of the manufacturers, the average weekly shipment of new vaccines should equal about 30 million doses, or more than twice the rate of weekly vaccinations if the administration pace remains where it is today.
That’s clearly unacceptable, and also unlikely to occur. Many states have said they could dramatically scale up throughput at their existing vaccination sites if available supply improved. The Biden administration is supporting states with 100 mass vaccination centers and has recruited a national network of pharmacies and health clinics to serve as vaccination hubs too. These combined efforts should continue the improvement seen over the past month.
The real test, however, is about to begin. With supply flowing, Americans who have been forced to wait for months for their shots will want to secure appointments fast, and inadequate supply will no longer be a legitimate excuse for making them wait even longer.
So the daily rate needs to go up, but by how much? A dynamic supply and throughput model is useful for providing such an estimate. Using such a model, this chart details the potential paths population-focused vaccination coverage could take under different daily vaccination scenarios.
Percentage of the US population vaccinated under different capacity scenarios
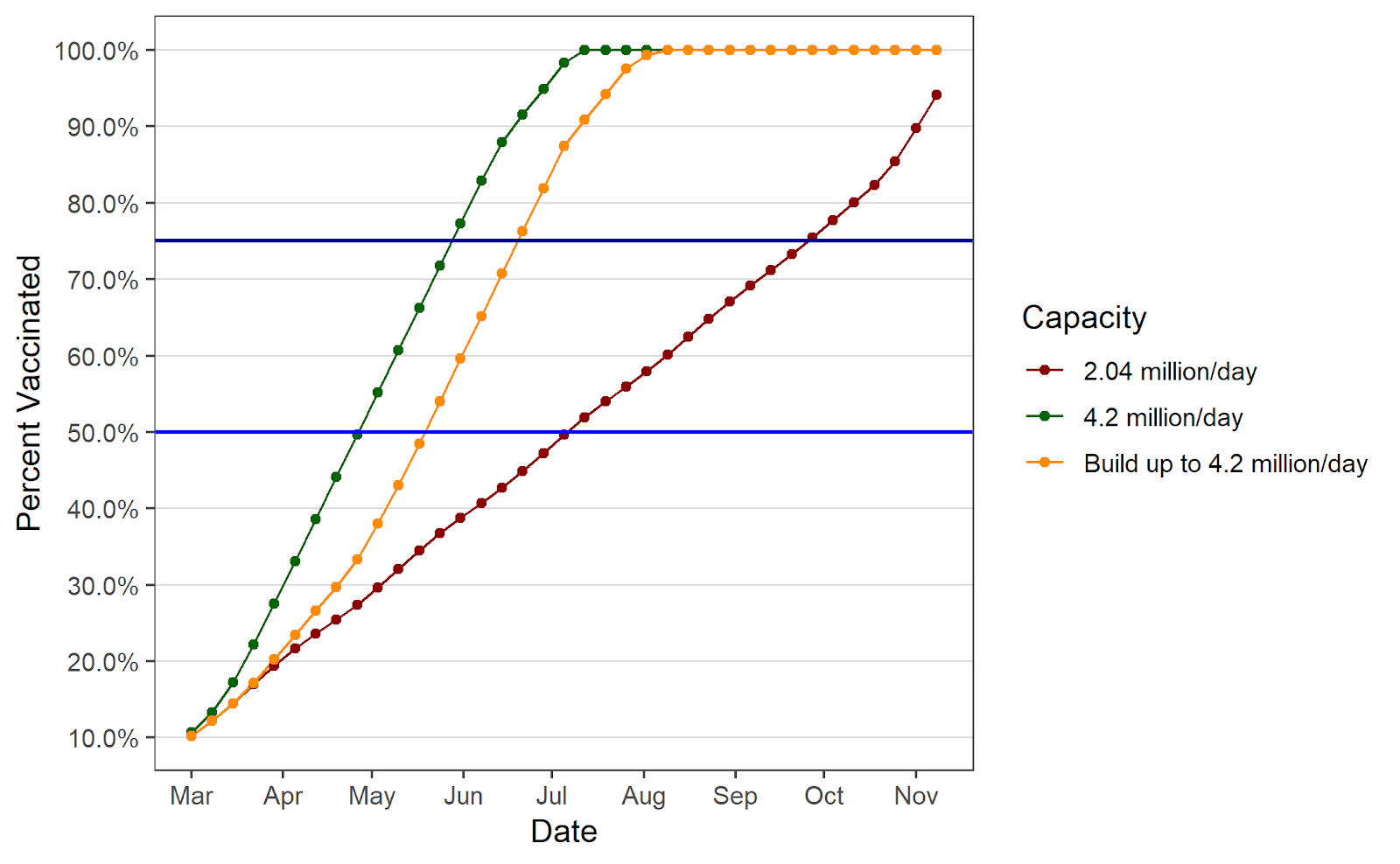
An important takeaway from the simulation is the inadequacy of maintaining 2 million shots per day in the coming weeks. Although it is a significant improvement over the rates observed in January and most of February, it will not be nearly rapid enough to make good use of the coming supply. According to the model, if the daily vaccination rate were to hold steady at the current rate of 2.04 million doses administered per day, the U.S. would not reach 50 percent of the population vaccinated until mid-July, and would not reach 75 percent until early October. Further, almost half (240 million) of the 500 million doses delivered would still be sitting as unused stock on May 31.
The daily vaccination rate that would take full advantage of the coming supply is 4.2 million per day. With such a rate, the constraint on vaccinations would be the pace of deliveries as opposed to the capacity to administer shots (the simulation assumes some of the supply is reserved for second doses of the Pfizer-BioNTech and Moderna vaccines). In this scenario, 50 percent of Americans could be fully vaccinated in less than two months’ time (by the end of April) and 75 percent by the end of May. Further, unused stock would not exceed 63 million until after that date. In addition, at this rate, assuming there is no vaccine hesitancy at all, 300 million people could be fully vaccinated by late-June.
Of course, this scenario is based on the unrealistic assumption that a daily rate of 4.2 million shots in arms is possible immediately. A more realistic assumption—that there is a steady ramp up to 4.2 million—only delays the progress modestly. With this assumption, 50 percent of the country would be fully vaccinated by late May, and 75 percent by late June, only four weeks behind the previous scenario. Additionally, unused stock would peak at a little more than 120 million doses in late May, before falling as vaccinations catch up to supply.
The real-world vaccination program will be far messier than a simulation, and it is to be expected that continued progress will occur. Still, these projections reveal one important point: It is not really possible to overshoot on building vaccination capacity. The U.S. government has succeeded in pushing producers to speed up the manufacturing process, but that success needs to be matched by continued improvement in the capacity to get doses administered. The imminent introduction of even more vaccination sites, and a more streamlined process for scheduling vaccinations, should speed up the country’s return to a status resembling normalcy.
Kieran Allsop is a research assistant at the American Enterprise Institute. James C. Capretta is a resident fellow and holds the Milton Friedman Chair at AEI. Scott Ganz is a research fellow in economic policy studies at AEI and an assistant professor at Georgia Tech’s School of Public Policy.