The Biden administration announced last week that it will recommend a third “booster” shot for Americans who have received either of the mRNA COVID-19 vaccines currently authorized for emergency use. (A recommendation for the viral vector shot developed by Johnson & Johnson, which is also available on an emergency basis in the U.S. market, will be provided at a later time, after data from its ongoing two-dose trial is assessed.) The prospect of hundreds of millions of third mRNA doses going into the arms of Americans before much of the world gets a first inoculation always was a possibility, but the recommendation to move forward came more quickly, and is broader in its reach, than many had expected. Its speed and breadth also are altering previous assumptions about the availability of excess U.S. supply for international donations.
A brief update on U.S. domestic needs and available inventory over the coming months, with the booster campaign fully included in the assessment, can clarify the options now available for further U.S. donations to the international vaccination effort. The U.S. still will be able to make a meaningful contribution to the global campaign—likely just more than 1 billion doses—but its contribution will remain far below what is required to vaccinate the worldwide population, and thus also falls well short of what would be required to substantially reduce the risk of more dangerous (and possibly vaccine-evading) variants emerging somewhere around the world.
The Current Status of Expected U.S. Consumption and Available Supply
The original Operation Warp Speed portfolio included a diversified mix of vaccine types, sponsored by six developers: the Pfizer/BioNTech collaboration, Moderna, Johnson & Johnson, Oxford/AstraZeneca, Novavax, and Sanofi/GlaxoSmithKline.
When the Phase III trials for the two mRNA candidates, from Pfizer/BioNTech and Moderna, showed them to be highly effective and very safe, the U.S. government moved quickly to make them the central pillars of its domestic vaccination campaign. To date, more than 345 million doses of the two mRNA candidates have been administered. Realistically, with the supply of the Pfizer/BioNTech and Moderna vaccines being sufficient to keep up with domestic demand, there is little prospect that any of the other candidates will get used in the U.S. market, at least during the current phase of the pandemic. A small exception is the J&J inoculation, which has been given to approximately 14 million people to date.
It remains possible that the vaccines developed by Oxford/AstraZeneca, which already is widely used globally, and by Novavax, which has yet to receive regulatory approval from any country (but may in the coming months), will still be relevant for the U.S. plans to support vaccinations around the world. The U.S. has contracts for purchasing hundreds of millions of doses from both of these manufacturers. The Sanofi/GSK candidate, however, failed its clinical trial, and a follow-on candidate is unlikely to be a relevant entrant in the market anytime soon.
Figure 1 provides an overview of use of how many vaccine doses must be held for U.S. consumption if everyone who is eligible is to be fully vaccinated, including with a booster shot. The current count of fully vaccinated Americans stands at 169.6 million, or 51 percent of the entire population and 60 percent of the population eligible for vaccination (those age 12 and older). In the coming months, it is highly likely that children over the age of 4 will also become eligible for vaccination. When they are included in expected future need, along with those currently unvaccinated and the need for booster shots, a total of 566 million additional doses will be required to fully vaccinate the U.S.
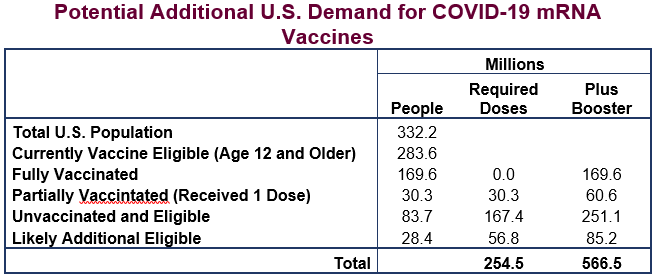
Not all of the doses set aside to vaccinate Americans will be used, as many millions of people will decline them, or will receive only one or two shots instead of the full three-shot regimen. Even so, the administration is likely to set aside sufficient doses for the entire U.S. population to get fully vaccinated until further efforts to improve take-up have run their course, and also to provide a reserve for contingencies.
Figure 2 provides a snapshot of the supply contracts with the mRNA manufacturers. The U.S. has domestic purchase orders in place with both Pfizer/BioNTech and Moderna to secure 500 million doses from each manufacturer. Pfizer/BioNTech have delivered 226.4 million doses so far, of which 202.1 million have been administered. Moderna has provided 173.1 million doses domestically (and an additional 64.9 million which have been donated to other countries), of which 142.5 million already have been used in the U.S. market. Thus, the manufacturers will be delivering a total of more than 530 million additional doses in the coming months, which tracks with the remaining U.S. consumption needs during this acute phase of the pandemic.

The Biden administration has stated that it will be active in supporting the global vaccination effort even as it places a priority on the domestic campaign. With its heavy reliance on the Pfizer/BioNTech and Moderna shots for domestic consumption, the government’s purchases of the other vaccines will be available almost entirely for international donations. As shown in Figure 3, that includes 300 million doses of the Oxford-AstraZeneca vaccine, 100 million of the Novavax shot, and roughly 72 million doses of the J&J inoculation.
Further, the U.S. government signed an agreement with Pfizer/BioNTech to purchase an additional 500 million doses of their vaccine for the express purpose of donating all of it to the international effort.

The U.S. is leading the way in terms of global vaccine donations, with almost 115 million doses given away to 73 different countries to date. Nearly 65 million of those doses have been Moderna, while more than 25 million have been the single-dose J&J shot.
All in all, there is likely to be more than 1 billion shots available from U.S. contracts with manufacturers that are not needed in this initial phase of the pandemic (it is possible that a need for annual boosters will disrupt this calculus next year).
While 1 billion vaccines may appear to be a significant contribution, it must be measured against the need, which is vastly greater. If every person needs three shots to be fully protected against COVID-19 infection, as public health authorities are now recommending for the American public, the implication is that more than 20 billion doses will be needed to vaccinate the entire global population. Nothing close to that will occur, but it shows that a donation of even 1 billion vaccines from the U.S. may be seen internationally as far less than what the moment—a true international crisis—requires.
James C. Capretta is a senior fellow and holds the Milton Friedman Chair at the American Enterprise Institute. Kieran Allsop is a research associate at AEI.




Please note that we at The Dispatch hold ourselves, our work, and our commenters to a higher standard than other places on the internet. We welcome comments that foster genuine debate or discussion—including comments critical of us or our work—but responses that include ad hominem attacks on fellow Dispatch members or are intended to stoke fear and anger may be moderated.
With your membership, you only have the ability to comment on The Morning Dispatch articles. Consider upgrading to join the conversation everywhere.