Happy Wednesday! Unless, of course, you had lots of money riding on the Supreme Court intervening in the presidential election and overturning the results in Pennsylvania. Then it is a very sad Wednesday for you indeed.
Quick Hits: Today’s Top Stories
-
The Food and Drug Administration released an analysis of the Pfizer/BioNTech COVID-19 vaccine Tuesday that concluded the vaccine was 95 percent effective, “met the prespecified success criteria,” and raised “no specific safety concerns.” The Vaccines and Related Biological Products Advisory Committee will meet tomorrow to discuss the analysis, and an emergency use authorization for the vaccine could follow within days.
-
Clinical trial data published yesterday show the AstraZeneca-University of Oxford COVID-19 vaccine to be 70 percent effective.
-
The Supreme Court yesterday denied a request by allies of President Trump to reverse the certification of the Pennsylvania presidential election. The order, issued without comment, featured no dissents.
-
The House of Representatives on Tuesday passed the $740 billion National Defense Authorization Act (NDAA) 335-78, a wide enough margin to override the veto President Trump has telegraphed. The bill, which directs military spending for the upcoming year, includes pay raises for troops and is filled with bipartisan priorities, but Trump has demanded an unrelated provision—the repeal of tech platforms’ Section 230 liability protections—be included.
-
President-elect Biden has reportedly selected Rep. Marcia Fudge to head the Department of Housing and Urban Development, and he plans to tap former Iowa Gov. Tom Vilsack as secretary of agriculture, a role Vilsack held for the entirety of the Obama administration.
-
Fourteen leaders at Fort Hood were suspended or relieved after a report released by the Army found the command climate at the Killeen, Texas, military base created a “permissive environment for sexual assault and sexual harassment.”
-
Gideon Saar, a former Cabinet minister under Israeli Prime Minister Benjamin Netanyahu, announced yesterday he is leaving Likud to form a new right-wing political party and challenge Netanyahu in next year’s election. “The Likud has changed and became a tool serving the personal interests of its leader, including in his criminal trial,” Saar said.
-
President Trump’s lawyer Jenna Ellis has reportedly tested positive for the coronavirus. She attended a White House Christmas party on Friday.
-
FireEye, a U.S. cybersecurity firm, disclosed last night its systems were hacked by “a nation with top-tier offensive capabilities.” The breach targeted the company’s “Red Team tools” that it uses to detect potential vulnerabilities in its clients’ security systems.
-
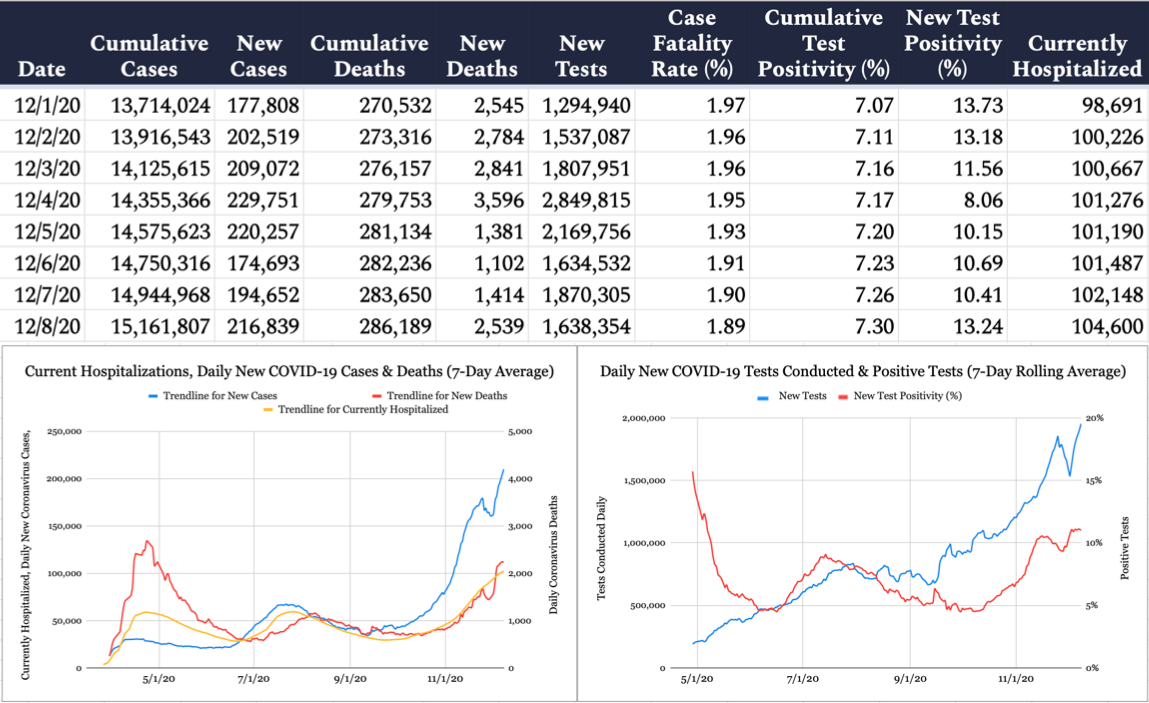
The United States confirmed 216,839 new cases of COVID-19 yesterday per the Johns Hopkins University COVID-19 Dashboard, with 13.2 percent of the 1,638,354 tests reported coming back positive. An additional 2,539 deaths were attributed to the virus on Tuesday, bringing the pandemic’s American death toll to 286,189. According to the COVID Tracking Project, 104,600 Americans are currently hospitalized with COVID-19.
A Veto-Proof Majority on the NDAA
Lawmakers in the House overwhelmingly approved the $740 billion National Defense Authorization Act on Tuesday in defiance of repeated veto threats from President Donald Trump.
It passed 335-78, with one member voting “present”—well above the two-thirds majority required to override a potential veto. The legislation, which includes a 3 percent pay raise for troops and provisions to rename military bases that honor Confederate soldiers, is packed with bipartisan priorities and is the product of months of negotiations.
The strong tally sends a message to Trump, who has in recent days said he would reject the compromise measure if Congress does not add unrelated language to repeal Section 230 of the Communications Decency Act, which protects online platforms from liability over user-submitted content. Some Republicans—most notably the president—argue the protections enable social media companies to silence conservative voices.
Trump has also taken issue with the legislation’s eventual removal of Confederate names and memorabilia from Defense Department properties, as well as limitations on troop withdrawals from Germany and Afghanistan.
“I hope House Republicans will vote against the very weak National Defense Authorization Act (NDAA), which I will VETO,” Trump tweeted Tuesday before the vote. “Must include a termination of Section 230 (for National Security purposes), preserve our National Monuments, & allow for 5G & troop reductions in foreign lands!”
GOP leaders were divided over how to handle his demands, with House Minority Leader Kevin McCarthy telling reporters that even though he supports the bill, he would not vote to override Trump’s veto. McCarthy’s statement earned praise from members of the conservative House Freedom Caucus, who urged their colleagues to oppose the bill and pursue Trump’s goals.
Republican Conference Chair Liz Cheney took a firm stance against the White House, saying plainly that Congress should override any veto on the legislation. House GOP Whip Steve Scalise didn’t recommend members vote one way or the other, although his office sent a notice just before the vote stating that House Armed Services ranking member Mac Thornberry urged a yes vote.
Only 40 Republicans voted against the legislation, joined by 37 Democrats and Libertarian Rep. Justin Amash.
Some members hope the formidable House vote, followed by similar results in the Senate, could persuade Trump not to follow through on his veto threat.
Senate Majority Leader Mitch McConnell suggested on Tuesday that the president could change his mind: “We don’t know for sure whether the bill will be vetoed or not,” he told reporters.
“We haven’t failed to pass the NDAA for 60 years,” the Kentucky Republican added. “If it comes over from the House, obviously I’m going to put it on the floor, and it’s my intention to vote for it.” Congress has approved the NDAA for 59 consecutive years—this year would make the 60th.
If Trump does stick to his guns, House Democratic leaders have said they would bring members back into session from an upcoming recess to override a veto if needed.
“If we don’t do our job, if we don’t pass this bill and exercise oversight, we are ceding authority to the executive branch—authority that is too great already,” House Armed Services Committee Chair Adam Smith said during floor debate. “Let’s not walk away from our biggest opportunity every year to exercise that legislative oversight. This is a good bill. If we don’t do this, we are not fulfilling one key aspect of our duties to our constituents.”
U.S. Inches Closer to COVID-19 Vaccine Approval
The United Kingdom celebrated its biggest milestone in the fight against COVID-19 yesterday when 90-year-old Margaret Keenan became the first person to receive the Pfizer vaccine outside of clinical trials. The second recipient was an 81-year-old Brit named—we are serious—William Shakespeare. Thousands of others across the U.K., primarily from high-risk groups, joined Keenan and Shakespeare in “getting the jab” throughout the day, and up to 800,000 immunizations are expected to be administered in the coming weeks.
The United States is lagging a few days behind its ally across the pond in vaccine approval and distribution (read Dr. Marty Makary in The Dispatch on why), but expect that to change this week.
The Food and Drug Administration released its initial review of the Pfizer-BioNTech vaccine candidate Tuesday, supporting the pharmaceutical company’s overwhelmingly positive findings in clinical trials. After evaluating the vaccination, the agency found it was “highly effective,” with a 94.8 percent success rate seven days after a second dose. For comparison, the annual flu vaccine reduces the likelihood of someone contracting influenza by only 40 to 60 percent.
The evaluation also found that the vaccine didn’t raise any “specific safety concerns,” though it identified common “mild to moderate” side effects: Injection site reactions were listed as the most common, followed by fatigue, headache, muscle pain, and chills. The report said more data are needed to make more conclusive determinations regarding the vaccine’s effect on asymptomatic infection and the ability to transmit COVID-19. “Demonstrated high efficacy against symptomatic COVID-19 may translate to overall prevention of transmission in populations with high enough vaccine uptake,” the report posits, “though it is possible that if efficacy against asymptomatic infection were lower than efficacy against symptomatic infection, asymptomatic cases in combination with reduced mask-wearing and social distancing could result in significant continued transmission.”
In an interview with BBC World News following the announcement, Dr. Anthony Fauci, the country’s leading infectious disease expert, said the biggest task facing U.S. health officials now is to be transparent in “letting the public know that in fact we are dealing with a safe and highly efficacious vaccine.” Fauci also predicted the U.S. will begin administering a mass vaccination program by the third or fourth week of December. The Vaccines and Related Biological Products Advisory Committee will meet tomorrow to discuss the analysis, and, pending its recommendation, an emergency use authorization for the vaccine could follow within days.
With Pfizer and BioNTech’s vaccine nearing approval, the focus has begun to shift to manufacturing and distribution. The companies originally planned to have 100 million doses ready to go by the end of 2020, but they slashed that number in half last month due to issues constructing a supply chain at breakneck speed. The New York Times reported Monday that Pfizer approached the Trump administration in recent months offering to sell the United States government more than the 100 million doses it agreed to earlier in the summer, only to be rebuffed. Scott Gottlieb, President Trump’s former FDA director and a current member of Pfizer’s board, confirmed the story in a CNBC interview yesterday.
“Pfizer did offer an additional allotment … to the United States government multiple times, and as recently as after the interim data came out and we knew this vaccine looked to be effective,” he said. “I think that the government made a bet that they are going to option, or advance purchase, vaccines from multiple manufacturers. They have agreements now with five or six manufacturers for about 100 million doses, each manufacturer. They want to spread those bets. I think they’re betting that more than one vaccine is going to get authorized, and they’re going to get more vaccines on the market. And that perhaps could be why they didn’t take up that additional 100 million option agreement.”
The result? Pfizer entered into agreements with other governments around the world, including last month’s deal to provide the European Union with 200 million doses. A source familiar with current negotiations between Pfizer and the U.S. government told the Times Pfizer can’t guarantee it will be able to deliver more than the already agreed upon 100 million doses—enough to immunize 50 million people—to the United States before June 2021.
As the Trump administration was building out Operation Warp Speed in the late spring and early summer, it of course did not possess the information we now have about the efficacy of various vaccines: It certainly made sense to hedge bets and enter into agreements with a variety of manufacturers using a variety of technologies. One senior administration official told reporters on Monday that pharmaceutical companies offering hundreds of millions of doses of a conceptual vaccine without proof it worked “was just not going to get the government’s money.”
Trump’s head of Operation Warp Speed, Dr. Moncef Slaoui, defended the administration’s decision in an interview yesterday with Good Morning America. “We selected six different vaccines to build a portfolio to manage the risk that some may work and some may not work, but also to ensure that as more than one would work, that we would accumulate vaccine doses from this portfolio of vaccines,” he said. “Now, in the summer, if somebody came to us and said, ‘Let’s buy more of this vaccine or that vaccine,’ no one reasonably would buy more from any one of those vaccines, because we didn’t know which one would work and which one may be better than the other.”
But if Gottlieb is correct in saying Pfizer approached the White House after the interim data was released showing its vaccines’ efficacy, the administration’s decision grows more puzzling. There are, of course, currently five other vaccine candidates that have a relationship with Operation Warp Speed—including Moderna, which recently reported clinical success and requested emergency use authorization (EUA) from the FDA. Johnson & Johnson’s chief scientist said yesterday the company expects results from its Phase 3 COVID-19 vaccine trial by January, and the U.S. also owns the rights to hundreds of millions of vaccine doses from AstraZeneca, Novavax, and Sanofi, if and when their candidates are approved.
Perhaps anticipating blowback for the way the decision has played out, President Trump yesterday signed an executive order—one that is almost entirely non-binding and in actuality more of a press release—reiterating that “it is the policy of the United States to ensure Americans have priority access to free, safe, and effective COVID-19 vaccines.” Slaoui was asked about the intent of the executive order: “Frankly, I don’t know,” he said. “I’m staying out of this, I can’t comment. … I don’t know exactly what this order is about.”
Best case scenario, the White House decision won’t end up mattering all that much as other companies’ vaccines are proven effective and approved. “I think we will have two [emergency use authorizations], Pfizer and Moderna, within the next 10 days, and then likely two additional EUAs in January, AstraZeneca and Johnson & Johnson,” Dr. Howard Forman, health policy professor at Yale University and clinician at Yale New Haven Hospital, told The Dispatch. “I think that even with the concerns about AstraZeneca, it is a very reasonable candidate and the least expensive, so it should not be dismissed.”
Worth Your Time
-
Yesterday, Bethany Allen-Ebrahimian and Zach Dorfman published in Axios their year-long investigation into a Chinese spy’s infiltration of California politicians from 2011 to 2015—most notably Democratic Rep. Eric Swalwell, whose longshot presidential bid ended with a thud last year. “Through campaign fundraising, extensive networking, personal charisma, and romantic or sexual relationships with at least two Midwestern mayors, [Chinese national Christine] Fang was able to gain proximity to political power,” the report claims, citing current and former intelligence officials. Swalwell is not accused of any wrongdoing, and he cut off all ties to Fang after being alerted to the intelligence community’s concerns. But the case “demonstrates China’s strategy of cultivating relationships that may take years or even decades to bear fruit,” Allen-Ebrahimian and Dorfman write. “The Chinese Communist Party knows that today’s mayors and city council members are tomorrow’s governors and members of Congress.”
-
In 2017, hundreds of Wisconsinites were forced from their homes to make room for a 20 million square-foot plant owned by the Taiwanese tech manufacturer, Foxconn. Local leaders in Mount Pleasant, Wisconsin, promised the project would result in 13,000 jobs and $10 billion in private investment by 2023; President Trump touted the coming factory as the “eighth wonder of the world.” But three years later, Tom Perkins reports in the Guardian that the project has more or less imploded. “Few jobs have materialized and Foxconn has not submitted new construction plans in over a year. The LCD screens that were supposed to be made there aren’t being built in its ‘factory,’ which is 20 times smaller than proposed and now zoned as ‘storage,’” he writes. The residents whose lives were uprooted are not pleased. “They demolished my house for this? A bunch of geese that sit on a hill?” said 37-year-old Sean McFarlane. “It’s upsetting. That’s where my old house was, and now it’s just nothing.”
-
In a piece for The Atlantic, Jordan Kisner reports on the ethical burdens being placed on health care workers as COVID-19 patients overwhelm hospitals. “This was a stress test for medical ethics, for distributive justice and the allocation of scarce resources,” one New York doctor said of the spring surge. “Simply put, there were more patients to be resuscitated than available personnel, much less equipment.” With limited guidance, doctors struggled to prioritize care, essentially having to decide who lived and who died. And countless ethical debates are about to spring up as vaccines are approved. “If we prioritize people who are more likely to contract and die from the illness—which is one common method of allocating vaccines—should Black, Latino, and Indigenous Americans be on the top of the list, given their documented vulnerability? Should the risks associated with being among the first to receive the vaccine be distributed more broadly? Should health-care workers get the first doses? What about schoolchildren, or teachers? Should we prioritize people most likely to die from the disease (say, the elderly) or those most likely to transmit it widely (say, college students)? Can a government compel some citizens to get inoculated? Should it?”
Presented Without Comment
Toeing the Company Line
-
Dispatch Live was back last night! Sarah, Steve, Jonah, and David broke down Biden’s cabinet picks thus far, how political parties can regain some of the power they’ve ceded, and the latest in the Trump campaign’s moonshot effort to overturn the results of the election. If you missed it (or want to rewatch it), you can catch the whole thing here!
-
In the latest edition of Capitolism (
), Scott Lincicome details just how global the effort to develop a COVID-19 vaccine was. Not only does the leadership of the various companies involved hail from Turkey, Germany, Greece, South Africa, Sweden, Lebanon, Morocco, France, Spain, and Israel, but global collaboration also made the record-fast approval process possible. “The BioNTech/Pfizer and Moderna vaccines rely less on a handful of government officials or any one nation than on the global flow of knowledge, capital, people, and goods—as well as the dense distribution networks and free market policies facilitating those movements—that existed long before we’d ever heard of COVID-19.”
-
Yesterday’s episode of The Remnant was the most ambitious crossover event in podcast history: Sarah brought her Advisory Opinions legal expertise to Jonah, and the two talked about the various constitutional provisions kicking into effect that are helping to slow down the chaos surrounding the November election results. Plus, an in-depth discussion of The Queen’s Gambit.
-
Up on the site today, Audrey has a piece diving into the Bitcoin boom—the price of one single Bitcoin hit $19,850 in recent weeks. She asks the obvious question: Can the cryptocurrency last? “Over the next few years, the cryptocurrency market is all but guaranteed to whipsaw between record highs and disappointing lows,” she writes. “Despite its volatility, however, investors remain optimistic that blockchain technology will soon revolutionize our understanding of the global economy and help create a world in which governments no longer retain a monopoly on currency.”
Let Us Know
With negotiations over another coronavirus relief package continuing on, Senate Majority Leader Mitch McConnell yesterday suggested the two sides drop the package’s more contentious provisions—Republicans’ desire for liability protections and Democrats’ push for state and local aid—to “pass those things that we can agree on, knowing full well we’ll be back at this after the first of the year.”
Senate Minority Leader Chuck Schumer rejected the notion almost immediately, saying funding for state and local governments has “broad bipartisan support,” while liability shields have “no Democratic support.”
Put your game theory hat on: What would you do if you were in McConnell and/or Schumer’s shoes, knowing your position could be strengthened or weakened by the results of next month’s runoffs in Georgia?
Reporting by Declan Garvey (@declanpgarvey), Andrew Egger (@EggerDC), Haley Byrd Wilt (@Byrdinator), Audrey Fahlberg (@FahlOutBerg), Charlotte Lawson (@charlotteUVA), and Steve Hayes (@stephenfhayes).







Please note that we at The Dispatch hold ourselves, our work, and our commenters to a higher standard than other places on the internet. We welcome comments that foster genuine debate or discussion—including comments critical of us or our work—but responses that include ad hominem attacks on fellow Dispatch members or are intended to stoke fear and anger may be moderated.
With your membership, you only have the ability to comment on The Morning Dispatch articles. Consider upgrading to join the conversation everywhere.