Happy Monday! The day before the election, we said we would give a shoutout to whichever reader correctly guessed the final Electoral College tally. Congratulations Denise Cote, you called it!
Quick Hits: Today’s Top Stories
-
Pfizer and BioNTech on Friday submitted an application for emergency use authorization for their coronavirus vaccine candidate to the Food and Drug Administration (FDA). “While we cannot predict how long the FDA’s review will take,” the agency said in a statement, “the FDA will review the request as expeditiously as possible, while still doing so in a thorough and science-based manner, so that we can help make available a vaccine that the American people deserve as soon as possible.”
-
The makers of a third leading COVID vaccine candidate, AstraZeneca and the University of Oxford, announced early Monday that their vaccine was between 62 and 90 percent effective (depending on the dosage administered) in preventing the disease in late-stage trials.
-
The FDA also issued an emergency use authorization for Regeneron’s antibody treatment to combat mild to moderate COVID-19 in patients who are at high risk of developing more severe symptoms.
-
Dr. Moncef Slaoui, top scientific adviser to Operation Warp Speed, said yesterday he expects Americans will begin receiving a COVID-19 vaccine on December 11 or 12, adding that enough Americans will be able to be vaccinated by May for life to begin returning to normal.
-
Bloomberg reports that President-elect Joe Biden will nominate longtime adviser Tony Blinken to be his Secretary of State. Blinken served as Deputy National Security Adviser and Deputy Secretary of State in the Obama administration.
-
Georgia’s Republican Secretary of State Brad Raffensperger and Governor Brian Kemp on Friday officially certified President-elect Joe Biden as the winner of the state’s presidential election. The Trump campaign immediately requested a recount—which it will not have to pay for because the final margin was within 0.5 percentage points—despite Georgia having just completed a hand recount last week.
-
A federal judge in Pennsylvania dismissed the Trump campaign’s lawsuit alleging widespread voting irregularities in mail-in ballots, allowing the state to certify its results today as scheduled. Sen. Pat Toomey of Pennsylvania released a statement shortly afterward congratulating President-elect Biden on his victory and asserting that the president had “exhausted all plausible legal options” in his state.
-
Sen. Rick Scott, Donald Trump Jr., and Andrew Giuliani (Rudy’s son) all announced over the weekend they had tested positive for the coronavirus. The younger Giuliani was in attendance at the Trump legal team’s press conference at the RNC last week.
-
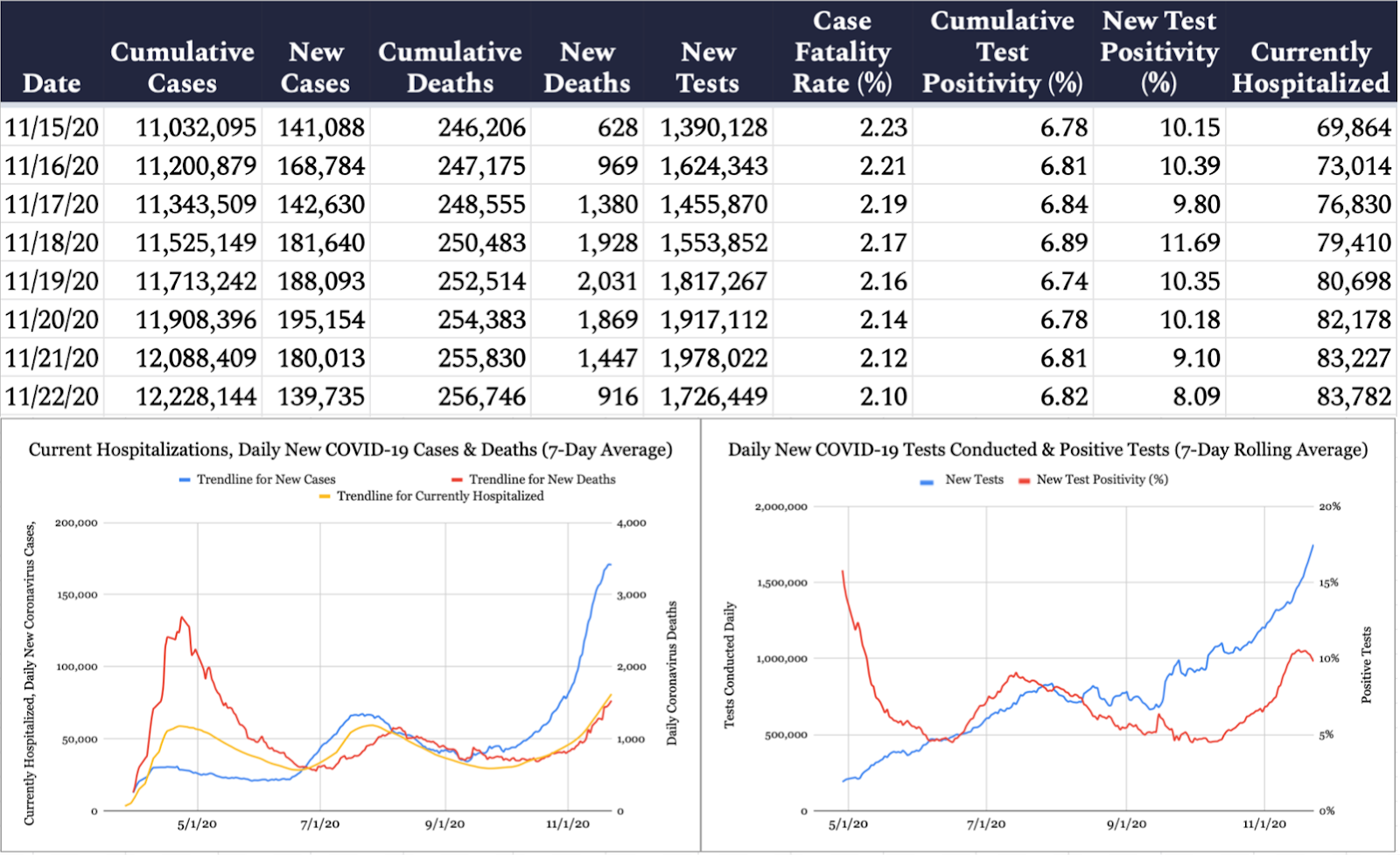
The United States confirmed 139,735 new cases of COVID-19 yesterday per the Johns Hopkins University COVID-19 Dashboard, with 8.1 percent of the 1,726,449 tests reported coming back positive. An additional 916 deaths were attributed to the virus on Sunday, bringing the pandemic’s American death toll to 256,746. According to the COVID Tracking Project, 83,782 Americans are currently hospitalized with COVID-19.
Trump’s Legal Team Has an Especially Bad Weekend
Rep. Steve Chabot—a longtime GOP member of the House from Ohio—was asked last week for his thoughts on the Trump campaign’s legal strategy. His response: “It sorta sucks.”
Judging by the numbers, it’s hard to argue with Chabot’s assessment; President Trump and his allies are now 2-34 in post-election litigation, and neither of those victories came close to changing the outcome of a single state, let alone the multiple states needed to reverse Joe Biden’s victory. To be fair, Trump dealt his lawyers a terrible hand by—as we noted Wednesday—claiming the election was stolen from him without having evidence to support the claim.
Broadly speaking, Trump’s plan to—as Jonah put it in his G-File—“steal an election he clearly and unequivocally lost” relied on three main pillars: Getting tens of thousands of legally-cast ballots tossed out in court, pressuring local officials and legislatures to overrule the will of the people in key states he lost, and keeping elected Republicans on message to present a unified front. Each element of the plan took a hit over the weekend.
The Legal Avenue
When we were last in your inbox on Friday, the president’s legal team had just delivered a 1.5-hour press conference alleging, among other things, a conspiracy theory involving Dominion voting systems, former Venezuelan president Hugo Chavez (who has been dead for seven years), and both Democratic and Republican candidates across the country paying “to have the system rigged to work for them.” Sidney Powell then expanded that conspiracy theory to allege that Georgia’s Republican Gov. Brian Kemp was “in on the Dominion scam” and that he rigged the state’s elections for Joe Biden and Kelly Loeffler.
In the days since, Trump’s lawyers—who described themselves as an “elite strike force” on Thursday—have fallen into complete disarray. An affidavit released by the Trump team on Michigan’s results began to crumble when it became clear the affidavit mistook Minnesota data for Michigan’s. After Giuliani’s son tested positive for COVID-19 on Friday, the entire legal team realized they had likely been exposed. On Sunday, Giuliani and Ellis issued a press release saying that Sidney Powell—whom Trump announced as a member of his “truly great team” last week—is “not a member of the Trump Legal Team.” (Last week, the Republican National Committee tweeted out a video of Powell that referred to her as a “Trump Campaign Lawyer.”)
But none of the above was the worst news of the weekend for Giuliani and Co. That came on Saturday night, when Judge Matthew Brann dismissed the Trump campaign’s last-ditch efforts to block the certification of the results in Pennsylvania, which is set to occur later today. The Trump campaign—which trails Biden by more than 80,000 votes in the state—alleged Republicans had been disadvantaged by certain counties allowing voters to “cure” their mail-in ballots. Brann—a conservative who had been involved in the NRA, the Federalist Society, and the Pennsylvania GOP prior to being nominated to the bench in 2012—did not hold back in his ruling.
In dismissing the Trump campaign’s action, he noted that the plaintiffs were essentially asking the Court to “disenfranchise” seven million voters. “One might expect that when seeking such a startling outcome, a plaintiff would come formidably armed with compelling legal arguments and factual proof of rampant corruption such that this Court would have no option but to regrettably grant the proposed injunctive relief despite the impact it would have on such a large group of citizens,” Brann wrote. “That has not happened. Instead, this Court has been presented with strained legal arguments without merit and speculative accusations, unpled in the operative complaint and unsupported by evidence. In the United States of America, this cannot justify the disenfranchisement of a single voter, let alone all the voters of its sixth most populated state. Our people, laws, and institutions demand more.”
Giuliani and Ellis attempted to spin the embarrassing rebuke in a statement claiming they welcomed Brann’s quick decision because they said it helps them in their strategy “to get expeditiously to the U.S. Supreme Court.” They filed an appeal yesterday, but—as Reuters legal reporter Brad Heath notes—the appeal they filed does not bring them any closer to the Supreme Court, but rather returns them to District Court where they would likely lose a second time.
The State Legislature Avenue
With Team Trump’s creative legal challenges stalling out, the president and his allies have begun eyeing Republicans in gubernatorial mansions and state legislatures as a means of flipping the requisite number of states into his column. The president repeatedly appealed to Gov. Kemp in Georgia, for example, and last week invited two GOP members of the Michigan legislature to the White House. Trump was rebuked by Republicans in both instances.
After election workers recounted five million ballots by hand, Georgia Secretary of State Brad Raffensperger and Gov. Brian Kemp certified the results on Friday afternoon. “The presidential outcome was remarkably close, but the new paper-ballot system, the strong election security and integrity mechanisms in place, and the audit and hand recount should combine to put to rest any doubts about the final outcome,” Raffensperger wrote in an op-ed. Kemp praised Trump as a “fighter” and called for another “audit” of the results, but said he had to “follow the laws of the constitution of this state” and certify the results.
Nor did Trump get the news he was hoping for out of what Press Secretary Kayleigh McEnany called a “routine” meeting with lawmakers in Michigan. “We have not yet been made aware of any information that would change the outcome of the election in Michigan and as legislative leaders, we will follow the law and follow the normal process regarding Michigan’s electors.” Michigan Senate Majority Leader Mike Shirkey and Speaker of the House Lee Chatfield wrote in a joint statement after the meeting, which they said focused on COVID-19 relief. “Michigan’s certification process should be a deliberate process free from threats and intimidation.”
Michigan’s canvassing board is set to vote on certifying the state’s results later today. CNN’s Jake Tapper reports that one of the GOP canvassers is expected to vote against certification, despite Biden’s 155,000-vote lead in the state. If the other GOP member of the board does as well, it could delay certification and move the process into the state’s judicial system.
The Political Avenue
Until this point, Republican elected officials have mostly kept mum on the Trump campaign’s legal proceedings, saying things like Trump is “within his rights” to pursue legal challenges or “the states should finish their work.” Trump-skeptical senators have already broken with the president, but some combination of time and the developments outlined above appears to be leading others to the same conclusion.
Shortly after Judge Brann released his decision in Pennsylvania, Sen. Pat Toomey—who is retiring in 2022—put out a statement of his own congratulating President-elect Biden. “With today’s decision by Judge Matthew Brann, a longtime conservative Republican whom I know to be a fair and unbiased jurist, to dismiss the Trump campaign’s lawsuit, President Trump has exhausted all plausible legal options to challenge the result of the presidential race in Pennsylvania,” Toomey wrote, also referencing the developments in Georgia and Michigan. “Joe Biden won the 2020 election and will become the 46th President of the United States.”
Toomey was joined by his Republican colleague Sen. Lisa Murkowski on Sunday night. She had already recognized Biden as President-elect, but yesterday called out Trump’s pressure campaign on state legislators, reiterating that “it is time to begin the full and formal transition process.”
Rep. Liz Cheney—the number three Republican in the House—said on Friday that, if Trump “cannot prove” his claims of widespread fraud, “he should fulfill his oath to preserve, protect and defend the Constitution of the United States by respecting the sanctity of our electoral process.” Rep. Kay Granger of Texas—no Never Trumper—said on Friday that “it’s time to move on”, adding that she has “great concerns” about the president’s efforts to overturn the election results.
Former New Jersey Gov. Chris Christie—who was the first 2016 presidential contender to endorse Trump and helped the president with debate preparation over the past few months—said yesterday it was time for Trump to call it quits. “I’ve been a supporter of the president’s. I voted for him twice,” Christie concluded. “But elections have consequences, and we cannot continue to act as if something happened here that didn’t happen.”
For now, the above remain the exception rather than the rule. Most GOP officials are still (publicly) in the wait-and-see camp, though some have begun calling for aspects of the Biden transition to begin as a contingency. Republican Gov. Larry Hogan said yesterday he is “embarrassed that more people in the party aren’t speaking up.”
Worth Your Time
-
Former FDA Commissioner Scott Gottlieb (whose Twitter is an invaluable follow for coronavirus commentary) writes in The Wall Street Journal about mask quality. While “there are still some shortages of medical masks,” he notes, “health-care workers have dedicated supply chains”—so consumers should try to upgrade their cloth masks. Surgical masks are roughly twice as protective as cloth, but “N95 or equivalent mask offers the best protection and, if used properly, will filter out at least 95% of infectious particles.” The key is making sure that the mask’s manufacturer is approved by the FDA. If Americans are diligent about choosing better masks, they can make a dent in transmission.
-
Laura Hazard Owen of NiemanLab surveyed 173 Facebook accounts to find out how many news stories people see on their Facebook feeds, and how much of that news is “fake news.” While caveating that her “sample size was small and shouldn’t be used to draw sweeping conclusions about Facebook news consumers overall,” Owen found that the most users she surveyed actually didn’t see much in the way of hard news, and very little that was labeled by Facebook as misleading or conspiracy-mongering. In the month of October, for example, a slight majority of the users studied by Owen (54 percent) did not see any news within the first 10 posts in their feeds at all. Additionally, although she saw some instances of partisan news, none of the 1,730 posts she looked at had been flagged by Facebook for including “false or disputed information.”
Presented Without Comment
Also Presented Without Comment
Toeing the Company Line
-
“The president of the United States is trying to steal an election he clearly and unequivocally lost,” Jonah writes in Friday’s G-File, channeling the white-hot rage of 1,000 suns. Trump “was pretty transparent about this long before the election,” he continues, noting the president spent months decrying mail-in ballots and telling his voters to go to the polls on Election Day itself. But Jonah reserves some of his harshest criticism for the elected Republicans encouraging Trump’s charade. “A serious party that cared about its long-term credibility, never mind the long-term credibility of our political system, would walk away from this burning septic tank en masse,” he concludes. “Instead it spends its days lobbing Molotov cocktails of flaming B.S. from its windows.”
-
Last week, GOP officials began pushing a clip showing Rev. Raphael Warnock—Sen. Kelly Loeffler’s Democratic opponent in January runoffs—delivering a sermon in which he said “you cannot serve God and the military” at the same time. The move comes just weeks after Republicans warned Democrats against attacking Amy Coney Barrett’s faith during the Supreme Court nomination process. In David’s Sunday French Press, he explores how and when to consider a politician’s faith when evaluating them.
-
Charles Koch and Brian Hooks joined Sarah and David on Friday’s episode of the Dispatch Podcast to discuss their new book, Believe in People: Bottom-Up Solutions for a Top-Down World. Koch and Hooks explain how finding common ground with people across the ideological spectrum has helped reorient their approach to public policy reform for the criminal justice system, education, and more.
Let Us Know
One of the best holidays of the year is just three days away! Given the circumstances, how are you planning to balance your family’s Thanksgiving traditions with public health guidance?
Reporting by Declan Garvey (@declanpgarvey), Andrew Egger (@EggerDC), Audrey Fahlberg (@FahlOutBerg), Charlotte Lawson (@charlotteUVA), James P. Sutton (@jamespsuttonsf), and Steve Hayes (@stephenfhayes).
Photo by Drew Angerer/Getty Images.







Please note that we at The Dispatch hold ourselves, our work, and our commenters to a higher standard than other places on the internet. We welcome comments that foster genuine debate or discussion—including comments critical of us or our work—but responses that include ad hominem attacks on fellow Dispatch members or are intended to stoke fear and anger may be moderated.
With your membership, you only have the ability to comment on The Morning Dispatch articles. Consider upgrading to join the conversation everywhere.