Happy Wednesday! Kanye isn’t the only one changing his name—Facebook CEO Mark Zuckerberg is reportedly planning to rebrand the social media giant next week like Google did with “Alphabet” a few years ago. That’ll fix everything!
Quick Hits: Today’s Top Stories
-
Total enrollment in U.S. schools fell by nearly 3 million from 2019 to 2020 according to data released by the U.S. Census Bureau on Tuesday. The trend was consistent across age groups, and just 52.4 percent of the under-35 population attended school last year—a 20-year low.
-
North Korea fired yet another ballistic missile into the Pacific Ocean Tuesday, just hours after the Biden administration reportedly “reaffirmed an offer to resume talks on [the county’s] nuclear weapons program.”
-
Days after the Association of Southeast Asian Nations excluded Burmese leader Min Aung Hlaing from a summit, Reuters reports that the ruling military junta in Burma released nearly 6,000 political prisoners arrested earlier in the year for protesting last winter’s coup. There are unconfirmed reports, however, that a handful of those released were immediately re-arrested.
-
A federal grand jury indicted GOP Rep. Jeff Fortenberry of Nebraska yesterday on charges that he concealed information and made false statements to FBI agents investigating illegal contributions made to his campaign by a foreign national in 2016. Fortenberry disputes that he lied to the agents, and he said he will fight the charges.
-
Members of the January 6 House select committee voted unanimously last night to recommend holding Steve Bannon—longtime adviser to former President Donald Trump who has been defying the committee’s subpoenas—in criminal contempt of Congress. If the full House approves the measure later this week as expected, it would be referred to the Justice Department for prosecution.
-
In an interview with the Wall Street Journal last night, Netflix co-CEO Ted Sarandos said he “screwed up” in how he communicated with Netflix employees upset by Dave Chappelle’s recent comedy special, but remained steadfast that the company will not remove the show from its platform. “We have articulated to our employees that there are going to be things you don’t like,” he said. “There are going to be things that you might feel are harmful. But we are trying to entertain a world with varying tastes and varying sensibilities and various beliefs, and I think this special was consistent with that.”
The State of COVID-19 Vaccine Boosters
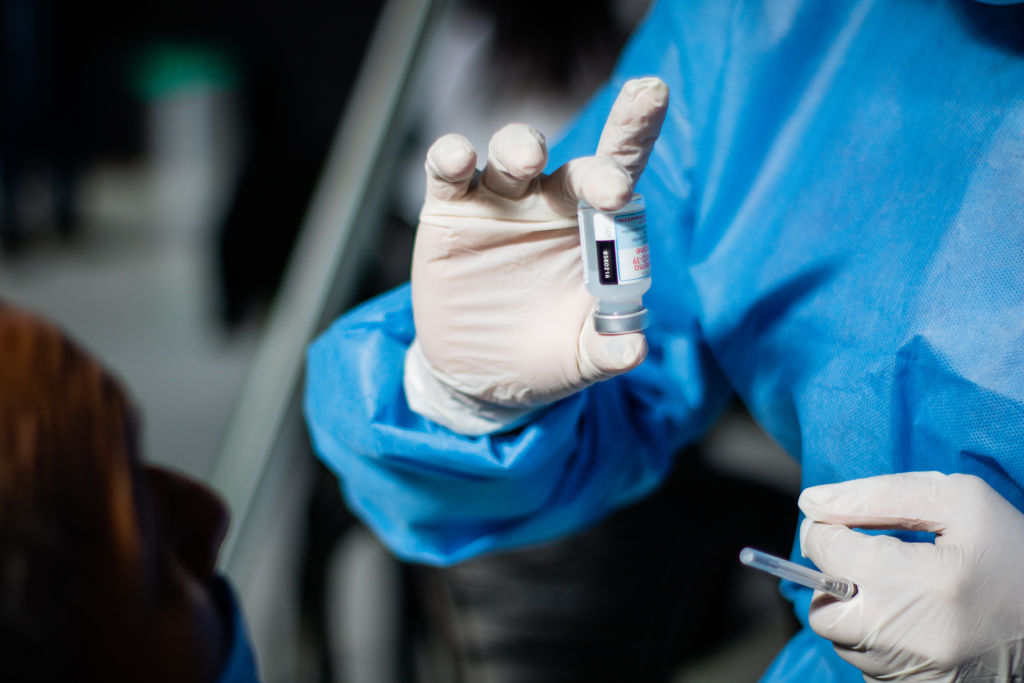
When we last wrote to you about COVID-19 vaccine boosters a few weeks ago, public health officials were torn over the need for them among the general, non-immunocompromised population. The Centers for Disease Control published a handful of studies showing that although the vaccines’ effectiveness against infection was reduced somewhat by both time and the Delta variant, the shots remained incredibly effective at keeping people out of the hospital—and alive.
Top Biden administration officials argued effectiveness against hospitalization and death could be the next domino to fall, but others weren’t so sure. “For protection against serious disease, really all you need is immunological memory,” Dr. Paul Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, told The Dispatch at the time. “You don’t necessarily have to have high titers, the virus’ neutralizing antibodies in your circulation. Immunological memory is longer lived.”
“I think [boosters] will be safe, and I think—without question—[they] will boost immunity,” he continued. “But my problem is do you need to do it now, or why couldn’t we wait until there was some evidence that you really needed to do it? … I just worry that it sends the wrong message out there that people aren’t protected and now will feel unprotected when they’re really not unprotected.”
A lot has transpired on this front, and it’s complicated. If you’re anything like us, you’ve seen a whole bunch of headlines about various boosters in recent days and aren’t entirely sure where things stand. There are different timelines for different vaccines, multiple stages of recommendation, authorization, and approval at various government agencies, and in a more recent development, regulators are planning to allow Americans to “mix and match” vaccines from different manufacturers.
Let’s break it all down.
Pfizer-BioNTech
If you received two doses of Pfizer-BioNTech back in the spring, there are decent odds you’re eligible to receive a booster shot already. Back in late September, the FDA and CDC authorized and recommended a third Pfizer dose—to be administered six months after the second shot—for those 65 and older, at high risk of severe COVID-19 infection, or who work or live in environments that result in “frequent” exposure to SARS-CoV-2. Among others included in the latter category are first responders, teachers and daycare workers, and health care workers. According to CDC data, almost 11 million people—including 15 percent of fully vaccinated senior citizens—have thus far taken them up on it.
Pfizer published a summary of clinical trial data in August concluding a booster dose “elicited significantly higher neutralizing antibody titers against the initial SARS-CoV-2 virus (wild type), and the Beta and Delta variants, compared to the levels observed after the two-dose primary series.”
Moderna
Having also gotten an mRNA shot, Moderna recipients are trending toward a similar outcome as their Pfizer counterparts—but they’re not there quite yet.
The FDA’s Vaccines and Related Biological Products Advisory Committee met last Thursday to evaluate safety and efficacy data for a third Moderna shot, and the body voted unanimously to recommend the FDA authorize a single 50-microgram booster—half the dose of the original regimen—for the same groups as Pfizer on the same timeline: Old, immunocompromised, or frequently exposed, six months after the second dose. The FDA is not legally bound by the committee’s recommendations, but it generally follows them—and likely will within days.
Moderna said back in May that clinical trials showed its booster dose “increased neutralizing titers against SARS-CoV-2 and two variants of concern (B.1.351, P.1) in previously vaccinated clinical trial participants.”
Johnson & Johnson
The Johnson & Johnson vaccine is unique among the three. It relies upon more traditional vaccination technology—not mRNA—and it was administered in a one-dose regimen rather than two. With months of data showing the J&J shot consistently offers slightly weaker protection than Moderna and Pfizer’s, some public health officials have in recent days called into question the earlier vaccine process.
“I think this frankly was always a two-dose vaccine,” Offit said at the Vaccines and Related Biological Products Advisory Committee on Friday. “It’s hard to recommend this as a one-dose vaccine.”
Because of these differences—and perhaps because it was by far the least widespread vaccine—the body voted 19-0 to recommend a J&J booster to everyone over the age of 18 who received a first dose. As with Moderna, this is not final, but likely will become so in a few days.
“[Johnson & Johnson] hasn’t performed quite as well as people hoped,” Dr. William Schaffner—an infectious disease specialist at the Vanderbilt University Medical Center who is currently a non-voting liaison to the CDC’s Advisory Committee on Immunization Practices—told The Dispatch. “The data has indicated that getting a follow up with either Moderna or Pfizer gives [people] a very big boost.”
The FDA’s advisory panel also endorsed all Johnson & Johnson recipients receiving a booster dose to increase their antibody levels. Johnson & Johnson has received less uptake than Moderna and Pfizer, though the company has argued that its shot continues to offer lasting protection against the virus. But some experts, including Schaffner, believe it should have always been a two-dose regimen.
Back in the spring, tens of millions of Americans desperate for a vaccine received whichever option was first available to them. But several months later—with vaccine supply now outstripping demand—people could theoretically supplement their first dose with a second (or third, for the mRNA vaccines) one of their choosing, and the FDA is reportedly working on a plan allowing people to do just that.
According to the CDC’s data on the effectiveness of the various vaccines against preventing infection, Johnson & Johnson’s vaccine was 66.3 percent effective, Pfizer’s vaccine was 95 percent effective, and Moderna’s vaccine was 94.1 percent effective.
Mix and match
The New York Times reported Monday that the FDA is on the verge of allowing Americans to receive a vaccine booster that is different from the one they originally received. The move would make it easier for states to administer shots, and is something state health officials have advocated.
In addition to assessing the data surrounding Moderna and Johnson & Johnson’s booster shots, the FDA’s advisory panel also heard from scientists from the National Institutes of Health (NIH), who presented a study showing that it is safe and effective for individuals to receive a booster from a different brand of vaccine. Experts told The Dispatch that—from a logistical and a health standpoint—mixing boosters is even preferable.
The study, from the NIH, has yet to be peer reviewed but the results are promising: It found that those inoculated with the Johnson & Johnson vaccine had increased levels of antibodies after they got booster shots from a different vaccine, such as Moderna or Pfizer, as opposed to sticking with Johnson & Johnson. With the Johnson & Johnson group, those who “mixed and matched” had antibody levels that were 50 times higher than those who also got a Johnson & Johnson booster. The latter had an antibody level that was about five times higher than without the booster. The study also found that mixing and matching booster doses for people who had gotten the Pfizer vaccine upped antibody levels. It will continue to follow the volunteers for a year.
Researchers checked the antibodies of 458 volunteers who received booster shots between four to six months after their original vaccination doses.
“The data in terms of mixing and matching of vaccines [shows that] getting the homologous vaccine as a boost would be less effective as getting the heterologous vaccine as a boost,” Offit told The Dispatch. “It’s significantly better and statistically better.”
So could boosters be in your future?
Schaffner said it may be likely as researchers learn more about the COVID-19 virus: “It is not at all unusual that our immune systems have to be reminded that they need to be stimulated to remember how to make the protection that we’re looking for from vaccines. … We were all looking down the road and saying this is going to be much more like flu, where periodically you have to get a booster.”
Worth Your Time
-
It’s all the rage nowadays to, well, rage against our fellow Americans who live life differently from us halfway across the country. But why? “No matter who you are, there’s probably a slice of America that’s just right for you, and a slice that’s just right for someone who’s your total opposite,” Kite & Key Media’s latest video notes. “It’s kind of amazing. We’ve let 50 different states fly their respective freak flags, and it’s all kind of worked—or at least, it used to kind of work. One of the reasons it’s getting tougher is research has shown that the more time we spend learning about people who aren’t like us from the media—as opposed to learning about people who aren’t like us from, you know, actually meeting those people—the more we tend to think they must be monsters. … Regional and partisan differences aren’t going to go away, but as the data shows they look more modest when we start dealing with real people instead of caricatures.”
-
Matt Labash picks up on this theme in his latest Slack Tide post about our collective “Anger Fever.” “Roughly a half decade or so ago, I started noticing that everyone began to believe that their political opinions were the most interesting thing about them,” he writes. “Achieving equanimity isn’t just a natural state, but a choice. These days, it very much involves swimming against the tide. You nearly have to choose not to get riled by all the manufactured outrages, Kabuki-theater conflagrations, and faux-Twitter fights that are conducted by catty people, for catty people. The rage merchants abound, and are all too willing to make a buck from stoking your anger and wet-nursing your resentments over ‘issues’ you’d never even heard of five minutes prior. Don’t be such an easy mark.”
-
On a lighter note, here’s Paul McCartney discussing how the Beatles wrote “Eleanor Rigby” in a piece for The New Yorker. “The song itself was consciously written to evoke the subject of loneliness, with the hope that we could get listeners to empathize,” McCartney notes. “Those opening lines—‘Eleanor Rigby / Picks up the rice in the church where a wedding has been / Lives in a dream.’ It’s a little strange to be picking up rice after a wedding. Does that mean she was a cleaner, someone not invited to the wedding, and only viewing the celebrations from afar? Why would she be doing that? I wanted to make it more poignant than her just cleaning up afterward, so it became more about someone who was lonely. Someone not likely to have her own wedding, but only the dream of one.”
Presented Without Comment
Also Presented Without Comment
Toeing the Company Line
-
David Drucker of the Washington Examiner made his first-ever Remnant appearance on Tuesday to discuss his new book on the 2024 Republican primary and the future of the GOP. The punditry is so rank you’ll be begging for mercy.
-
David’s latest French Press (🔒) is a rumination on Democratic Sen. Kyrsten Sinema and her unique willingness to buck partisan politics. “The intriguing thing about Sinema,” he writes, “is that she illustrates both the promise and peril of a candidate who reflects ‘disgust’ and ‘frustration’ not so much with the partisan enemy, but with polarized politics itself.”
-
In Tuesday’s Uphill, Haley and Harvest break down the January 6 select committee’s latest push to secure testimony from longtime associates of former President Trump, including Steve Bannon.
-
This week’s Sweep has a little bit of third-party dreaming and a whole lot of Virginia gubernatorial handicapping. “Virginia has moved from purple to reliably blue since 2008,” Sarah writes. “McAuliffe should win so if he does by 3 or more points, it’s a dog-bites-man story and there’s not a whole lot to take away from it except that nothing going on right now (Biden’s approval rating/COVID/inflation) is dramatically altering the political landscape.”
-
On the website today, Wang Xiyue and Behnam Ben Taleblu argue that there is a “direct line” between Biden’s botched withdrawal from Afghanistan and the administration’s efforts to renew the Iran nuclear deal. “Both are borne out of a misguided belief that the region no longer matters and that the best thing Washington can do is de-escalate,” they write.
Let Us Know
In light of that “Eleanor Rigby” piece from Paul McCartney, what’s another song you really want to hear the story behind?
Reporting by Declan Garvey (@declanpgarvey), Andrew Egger (@EggerDC), Charlotte Lawson (@lawsonreports), Audrey Fahlberg (@AudreyFahlberg), Ryan Brown (@RyanP_Brown), Harvest Prude (@HarvestPrude), and Steve Hayes (@stephenfhayes).






Please note that we at The Dispatch hold ourselves, our work, and our commenters to a higher standard than other places on the internet. We welcome comments that foster genuine debate or discussion—including comments critical of us or our work—but responses that include ad hominem attacks on fellow Dispatch members or are intended to stoke fear and anger may be moderated.
With your membership, you only have the ability to comment on The Morning Dispatch articles. Consider upgrading to join the conversation everywhere.