One of the problems with a novel virus, such as the coronavirus, is that doctors must race to figure out what medicines might work to treat patients. Over the course of this pandemic, we have seen several potential treatments receive a great deal of attention, including hydroxychloroquine and, more recently, remdesivir.
As a doctor myself, I’d much rather wait for the results of randomized, controlled studies before prescribing any medication. But as a front-line hospital provider working in a region handling the worst of the COVID-19 pandemic in the US, I don’t have that luxury. And so I—and my colleagues—have been using treatments that haven’t gone through that whole process. This is not to say, however, that there is no evidence for them.
Hydroxychloroquine quickly became controversial because it was highlighted repeatedly by President Donald Trump in his daily press briefings, and because news reports about early studies of the drug have questioned its possible efficacy.
Meanwhile, remdesivir—an antiviral drug being produced by Gilead Sciences—has reportedly yielded a “clear-cut” benefit in trials. Dr. Anthony Fauci said recently that it could be “the standard of care” going forward. It is now approved by the FDA for clinical use under an “Emergency Use Authorization,” and the federal government is coordinating distribution to the states of available doses.
What’s often missing from news coverage of these medications is a thorough explanation of what they do or how they work. As a physician who has used some of these treatments in clinical practice, I believe it’s useful to look at how the disease attacks the body and how the various potential treatments work, and also address major risks and side effects.
First, a brief overview of the disease process. Clinical COVID-19 disease has essentially two phases, and different treatments target different phases. The first is the “viral response phase,” where the disease is caused mostly by the direct effects of the SARS-COV2 virus, as it invades host cells and takes over the cell to make copies of itself. Any antiviral treatment is targeted to this early phase. As the disease progresses, the “host inflammatory response” starts to predominate. In the sickest patients the body starts to attack its own cells and tissues rather than just fighting off the virus. Various anti-inflammatory treatments are used this phase.
Now let’s look at what we know about a few various potential treatments.
Remdesivir
This drug first broke into the news in late April, based on a leaked video of industry researchers talking about its upsides.
The recent comments from Fauci—and the FDA’s Emergency Use Authorization (EUA)—are based on both preliminary results of a study sponsored by the manufacturer, Gilead, and a randomized, placebo-controlled study run by the National Institute of Allergy and Infectious Diseases (NIAID) that found remdesivir “had a 31% faster time to recovery than those who received placebo.” Patients who received remdesivir recovered in 11 days, compared with 15 days for those who received placebo.
The study also found suggestion of reduced mortality rates, but while the faster recovery time was considered statistically significant, the survival benefit was not.
Remdesivir was originally created as a possible treatment for Ebola, but it was found to be generally ineffective in that role. It has also been researched as a treatment of the original SARS and MERS, both caused by other coronaviruses.
The drug is an “RNA polymerase inhibitor,” which means that it works to keep the virus from making more copies of its RNA. Viruses consist of a core of genetic material (DNA or RNA) and some proteins, all covered in a coating called a capsid—think of a sugar candy shell covering a gummy snack. Viruses must “hijack” a host cell’s machinery in order to produce more copies of themselves.
To do this, the virus must attach to the surface of the host cell and inject its genetic material—RNA in the case of SARS-COV2—into it. This, in turn, tricks the cell into making copies of its DNA or RNA and assembling all that into more viruses that eventually escape the host cell and go infect more cells.
To make an analogy to technology, it’s as if some hacker sent, well, a virus, into a computer that was programmed to do nothing but make copies of itself, wrap it in a deceptively named .zip file, and send it out to everyone in the address book of the computer owner.
Remedesivir is currently only available—even under the EUA—to patients hospitalized with some degree of breathing difficulty; one recommended cut off is an oxygen saturation under 94 percent. However, most antiviral medications work best when given early in the course of disease. Reportedly, Gilead’s own studies have shown that patients given remdesivir earlier had better outcomes than those who received it later.
This dilemma can be overcome once the medication is more easily available than it is right now. As I write this, I have not personally given this medication to any patients, although I hope that will change in the next week or two.
The COVID Cocktail
This treatment doesn’t include any alcohol (sorry) but it does include the much-debated hydroxychloroquine. Patients admitted to New York City hospitals in late March onward were often provided with a “cocktail” of four drugs (or, to be specific, two drugs and two vitamins): hydroxychloroquine, azithromycin, vitamin C, and zinc.
Hydroxychloroquine
How it works: When the virus enters cells in the lungs, it is initially “swallowed” up in a pouch within the cell, called an endosome. The virus then needs to get out of this pouch to release genetic material into the cell. Once the virus replicates inside a host cell, its pieces get made and processed by the host cell’s manufacturing system, and results in the release of the new viral proteins within more endosomes. These endosomes are usually acidic. But hydroxychloroquine (HCQ), a weak base, can make the contents less acidic. As viral protein configuration is dependent on a certain pH, raising the pH will make the proteins unfold, making them nonfunctional.
HCQ’s known anti-inflammatory components (used primarily these days to treat lupus and other rheumatic diseases) may also help decrease the severity of disease. So, if HCQ can fight the disease process in both the viral response phase and inflammatory response phase, then it would seem to offer the best of both worlds.
Unfortunately, there are yet no results on this treatment from the gold standard of studies, the large scale randomized clinical trial. Two early studies (one in France, one in China) didn’t actually test the drug’s efficacy—that is, did it make anyone better?—but viral clearance—Can you find the virus in patients after the treatment?
Three recent studies of patients receiving HCQ have cast major doubts on its efficacy. While none are randomized placebo-controlled studies, both involve much larger numbers of patients than the first studies.
A recent study of patients treated by the Veterans Administration (VA) that found no evidence of actual clinical benefit was published before peer review on medRxiv, and included 368 VA patients treated with either HCQ alone, HCQ and azithromycin, or neither. The death rate was higher in patients who took the drug than without, while rates of needing ventilation were slightly less conclusive. One major limitation of the study was that due to the nature of the VA population, the patients were all men with a median age of 65, of which many were African American and/or obese, and therefore, already predisposed to poor outcomes from the disease itself.
A more recent study published in the New England Journal of Medicine reviewed 1,376 cases of COVID-19 patients treated in a major NYC hospital system. This analysis showed no actual benefit of either HCQ or azithromycin.
The raw numbers even suggested that the risk of death or intubation was twice as high in patients who received HCQ than those who did not. Adjusting for how HCQ-treated patients were sicker and at higher risk for poor outcomes, the study concluded that there was no evidence that it harmed patients, but no evidence that it helped, either.
Finally, a study published just Monday in the Journal of American Medicine similarly showed no lower mortality rates among those treated with HCQ, with alone or with azithromycin.
There are also ongoing randomized clinical trials of hydroxychloroquine, both with and without azithromycin, and a large Novartis study is planned soon. There still may, eventually, be some data found that shows benefit from HCQ, but clinical practice has started to shift away from using it outside a clinical trial. I, myself, am much more skeptical than I was two weeks ago.
Azithromycin
Azithromycin is primarily marketed as an antibiotic. But there actually is some data that azithromycin may have antiviral effects, and may work against COVID-19 in the same way hydroxychloroquine does. Early on when COVID-19 testing was not readily available, it was given in case there was either a bacterial pneumonia instead of the virus, or bacterial superinfection along with the virus. But it was also used, not as an antibiotic, but an anti-inflammatory medication, as azithromycin has also been used for years to treat patients with exacerbations of chronic obstructive pulmonary disease (COPD).
Vitamin C and zinc
Why give these supplements? Well, there is some data that intravenous vitamin C may decrease mortality in patients with acute respiratory distress syndrome (ARDS), a condition that often results in ventilator use in patients with COVID-19. However, intravenous vitamin C is not always available, and I have seen oral vitamin C being used instead. These are very high doses. Zinc was shown in a study on the original SARS virus (the one that causes COVID-19 is SARS-COV-2) to possibly inhibit viral RNA replication.
So that’s a very brief overview.
Also, while it has been making headlines, let me discuss the main risk of this cocktail: arrhythmias. Both HCQ and azithromycin can predispose to EKG abnormalities, and specifically, a fatal rhythm called a “multifocal ventricular tachycardia.” I prefer the French term, “torsades de pointes.” You don’t want to be in this rhythm. If you are, you should hope a code team is on the way with a defibrillator. If you are in the hospital taking these medicines, then you’ll probably be on a heart monitor, or at least getting daily EKGs.
Convalescent Plasma
You may have seen ads asking for volunteers who have recovered from the virus to donate plasma. The concept is pretty simple. Once someone gets COVID-19 and recovers (or convalesces), we expect them to have antibodies against the virus. So, why not take their plasma (the liquid part of the blood), and give it to patients who are sick with the virus now, allowing those antibodies to fight the virus? This strategy has been used to treat the original SARS, the 2009 “swine flu,” Ebola, and other viral infections. Some small studies have shown benefits. A number of clinical trials are underway, including a major one run by the Mayo Clinic.
A recent Wired article explains the logistics and also gets into the pros and cons:
One of the upsides to trying convalescent plasma as a therapy is that it’s readily available from all those donors, and can be given to a patient as soon as 36 hours after being collected. But there are still many practical unknowns when it comes to using it for Covid-19. Should it be used only to help very sick patients, or should it be administered earlier in the course of the infection, before patients need to be put on a ventilator? How much do people need?
Summary
All this being said, the bottom line is that there currently are no medications that have been conclusively proven to be effective in not only treating COVID-19, but reducing mortality—that is, the number of deaths. Remdesivir does have some data signal that it could, but this is not conclusive.
Unfortunately, the way HCQ was touted by the president and others gave the impression that it was a surefire cure with little risk; and I have been answering many questions from patients and family members, trying to explain this is not the case. I have had to explain that about remdesivir, plasma therapy, and some other therapies as well.
Many physicians have refused to prescribe HCQ, and many others would not do so for COVID-19 outside the context of an ongoing clinical trial.
While I respect those colleagues, I personally side with those who believe “we don’t have the luxury of waiting for those big, definitive randomized trials,” and that “given … the dire situation we find ourselves in, it may be very reasonable to use this drug with a relatively good safety profile. But, if we want to use hydroxychloroquine … we need to tell the public the truth: We’re not too sure it will work, and it may even be harmful.”
I graduated from medical school in 2006, so I’ve seen a lot through the years, including swine flu in 2009. I have cared for thousands of patients, including hundreds of the critically ill and dying. I can honestly say that never before in my career have I had to deal with anything comparable to this current pandemic.
Although the situation on the front lines has improved much since the peak in early-to-mid-April, I haven’t forgotten the feeling of despair that came over me so many times, when I realized a patient was beyond help, and I’d soon be calling a family member to say “I am sorry, but I have to tell you …” I haven’t forgotten feeling unworthy of being called a hero, when it seemed all I was doing was watching patients die. In that atmosphere, I find it hard to fault any health care providers for decisions made to use treatments that we might not consider in a more standard setting.
Finally, while I am keenly aware, and distressed, by the populist tendency to rebel against the proclamations of experts, I also don’t let us, the experts, off the hook on this. I have always considered it part of my job description not only to treat patients, but to educate them about their conditions, and to be honest when there is uncertainty in these matters, as is the case here.
I expect we will be learning more about the myriad ways that COVID-19 affects the body, and other possible pathways for treatment, for weeks and months to come. And I will use that new data that comes in to weigh the risks and benefits in coming up with treatment plans for my patients.
DISCLAIMER: The contents of this article are for informational purposes only. It is not an attempt to substitute for the practice of medicine nor as a substitute for the provision of any medical professional services. This information should not be used to replace, substitute for, or overrule a qualified medical professional’s judgment.
Akino Yamashita, MD, is a physician board-certified in internal medicine. She currently practices in New York and New Jersey.
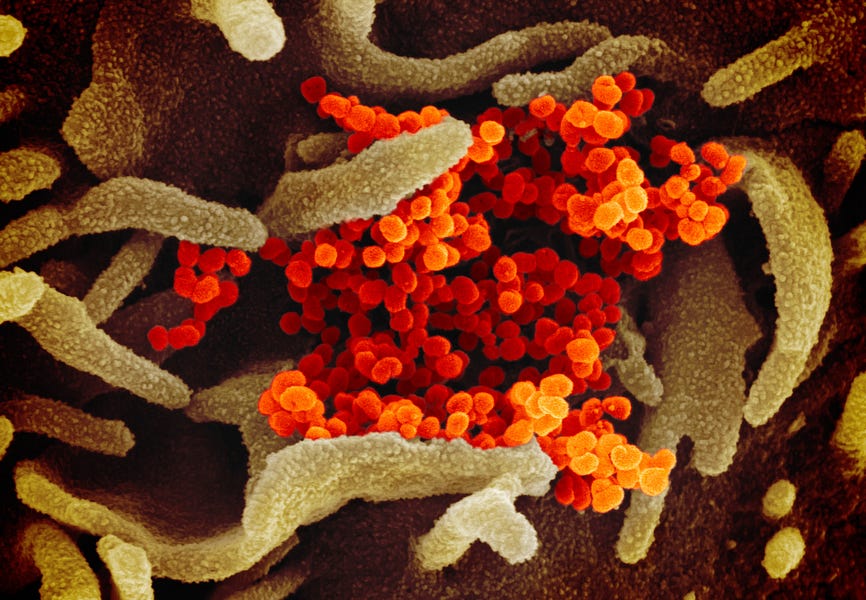
Photograph of coronavirus cultured in a lab by Universal Images Group/Getty Images.






Please note that we at The Dispatch hold ourselves, our work, and our commenters to a higher standard than other places on the internet. We welcome comments that foster genuine debate or discussion—including comments critical of us or our work—but responses that include ad hominem attacks on fellow Dispatch members or are intended to stoke fear and anger may be moderated.
You are currently using a limited time guest pass and do not have access to commenting. Consider subscribing to join the conversation.
With your membership, you only have the ability to comment on The Morning Dispatch articles. Consider upgrading to join the conversation everywhere.