It’s been exactly one month since President Trump, touring CDC headquarters in Atlanta, promised that the United States would not be caught flat-footed by the looming threat of coronavirus, saying that “anyone who wants a test can get a test.” As the world changed dramatically over the following weeks, the U.S. testing operation lurched into motion—belatedly, but with admirable speed. Yet even now, with our testing operation chugging along, we remain in many ways as far from understanding the scope of the virus’s spread in America as we were the day the president made that proclamation.
The simplest way to assess our own tracking of the coronavirus outbreak is to benchmark our own testing efforts so far. To do so we must consider two important data points. The first is the raw number of people tested: More than 1.7 million already, according to the COVID Tracking Project, with another 130,000 or more coming in every day. This is the number the White House has focused on: President Trump has bragged on multiple occasions that the U.S. has now done “far more testing than any other nation, by far.” It amounts to about 5,400 tests per million U.S. citizens—a remarkable mobilization given our sluggish start.
But to judge whether our testing regime has caught up to the virus, there’s another important benchmark to keep in mind: The rate at which the tests administered are coming back positive. This helps us to understand how widely we’re casting our testing nets. When there are only enough testing kits to check patients with obvious, severe symptoms, the rate of positive cases is likely to be disproportionately high; if we start having enough kits to test more borderline cases, we should expect that rate to creep down. Unfortunately, that positives number has been moving mostly in the wrong direction: from the low teens in early March to above 20 percent most days now.
The central difficulty: What matters is not so much the uptick in testing numbers as whether tests are being introduced into the population more quickly than the virus itself. One doctor we spoke to put it this way: “If we’re doubling our test production every week, but we’re doubling our number of cases in the country every two to four days, we’re never going to catch up.”
To this huge, all-encompassing epistemological problem can be added a slew of smaller ones. There’s the fact that the test currently in ubiquitous use, which relies on a process called polymerase chain reaction to detect viral RNA in a sample, has several downsides. PCR tests are relatively labor-intensive on the laboratory side, which has led to a substantial backlog of kits awaiting testing at many U.S. labs. Several doctors and COVID patients told The Dispatch that COVID-19 tests, which ideally could be turned around in as little as two days, are instead taking eight or 10 in many places, as labs slog through the logjam.
And while the PCR COVID test virtually never turns out false positives, many doctors are reporting a high level of suspected false negative results—where a test does not show a COVID infection despite a person meeting the symptom profile.
Then there’s the fact that the decision about who should be tested and when is extremely decentralized, with individual states, hospitals, and doctors making their own decisions about the best way to allocate limited resources. In areas that are already feeling the strain, medical providers just trying to keep their heads above water can sometimes take precedence over prioritizing tests.
“Most doctors would agree about what level of suspicion to have in terms of the likelihood of any given patient having COVID,” Dr. Andrew Cureton, who runs an urgent care practice in Northern Virginia, told The Dispatch. “But since there isn’t any proven directive treatment for COVID, whether a given patient should be tested largely depends on the reason the doctor might want to test them. … Between the burden of getting tested, the long delay in getting test results, and the lack of enough public health workers to follow up on the contacts of positive cases, knowing for certain a patient has COVID makes less of a difference than it otherwise might.”
Throw in the fact that the Centers for Disease Control and Prevention estimates that as many as a quarter of all contagious carriers never show symptoms at all, and you begin to get a sense of the scale of the problem here. Every day, the United States substantially improves its coronavirus testing capacity—but in doing so we are still getting no closer to mapping the disease’s full spread.
The point is not that our testing regime has failed. If and when our stringent national distancing measures succeed in dramatically slowing the rate of new coronavirus infections, it will once again become critical for medical providers to resume aggressive contact tracing—aggressively tackling each new diagnosis to ferret out all other people that person might have infected, then speedily testing them too. We missed the opportunity to curtail the initial outbreak. If we continue to scale up our testing operations, we can at least hope that next time we’ll be ready.
In the meantime, there are other technologies that may soon provide desperately needed answers to still-outstanding questions about the disease’s spread. One in particular—“Just how large a portion of the American public has already been exposed to the virus?”—is the biggest remaining blank that will shape what our policy response looks like as the crisis drags on in the weeks ahead. And it’s a question that’s unlikely to be solved primarily through PCR testing at all, but through an entirely different test that’s just starting to come online.
A number of U.S. companies are currently at work developing serology tests, which differ from PCR tests in several key respects. Serology tests don’t check for the presence of the coronavirus itself; rather, they analyze a blood sample for antiviral antibodies, evidence that a person has previously come into contact with the virus. The FDA authorized hospitals to start running such tests late last week. Over the weekend, The Dispatch spoke to one patient who was administered such a test and received his results that same day, all while still waiting on his PCR results from more than a week before.
From an individual medical perspective, the serology test is less useful than the PCR test: There’s a period of lag after infection before the body starts producing antibodies, meaning the test is more reliable to determine who has had the coronavirus than it is to determine who has it. By the same token, a serology test offers no information about whether a person is still “shedding” the coronavirus—in other words, whether they remain contagious.
From a public health standpoint, however—where the question of who had the virus matters just as much as the question of who has it—serology tests are likely to prove incredibly useful. For people who were never seriously ill enough to seek a PCR test, they will be able to offer quick confirmation that the bug they suffered was indeed the novel virus. Because it’s a simple blood test, serology kits will likely be able to be self-administered. They also have the potential to be processed on-site, rather than requiring a sample to be schlepped to a lab.
A reliable, mass-producible serology test, in other words, would give authorities a weapon they have heretofore lacked: the ability to conduct random population testing to get a sense of how widely the virus has previously spread. This information will be crucial in determining how quickly we can safely reopen portions of our economy, because it would determine with more clarity than ever before how far along the infection curve we actually are.
“It’s really important from a population health standpoint, and could give us tremendous clarity and visibility of what’s going on,” Dr. Howard Forman, a health policy professor at Yale, told The Dispatch. “Because it would completely change our policies and how we manage things if we were to discover that New York City, 30 percent of the population is positive for antibodies.”
If reliable antibody tests revealed far greater-than-expected spread, this would counterintuitively be good news: It would indicate that the percentage of cases that became seriously dangerous were far lower than previously believed, that hospitals in hotspots are currently going through the worst of the epidemic rather than bracing for still-worse horrors weeks or months down the road, and that social distancing measures could begin to be relaxed safely far sooner than otherwise.
“If it’s 5 percent, then the game’s very different,” Forman said. “Everything’s back to where we were. We have to keep things pretty hard locked down for a while.”
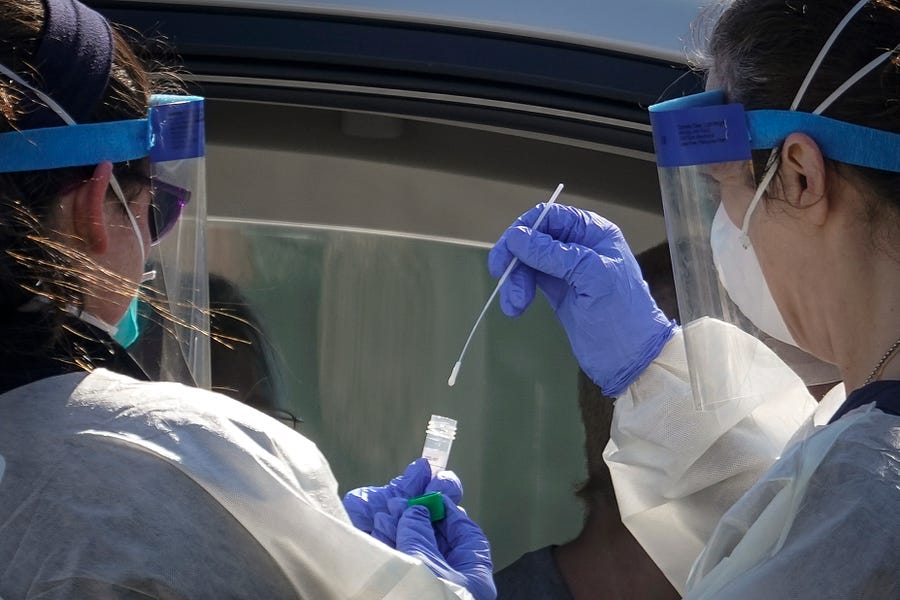
Photograph of a drive-up testing site in Washington, D.C. by Drew Angerer/Getty Images.









Please note that we at The Dispatch hold ourselves, our work, and our commenters to a higher standard than other places on the internet. We welcome comments that foster genuine debate or discussion—including comments critical of us or our work—but responses that include ad hominem attacks on fellow Dispatch members or are intended to stoke fear and anger may be moderated.
With your membership, you only have the ability to comment on The Morning Dispatch articles. Consider upgrading to join the conversation everywhere.